What is the basophil histamine release assay (BHRA)?
The basophil histamine release assay (BHRA) is an in vitro diagnostic test that can be used for different purposes. The assay can test for different indications within allergies and chronic spontaneous urticaria (CSU).
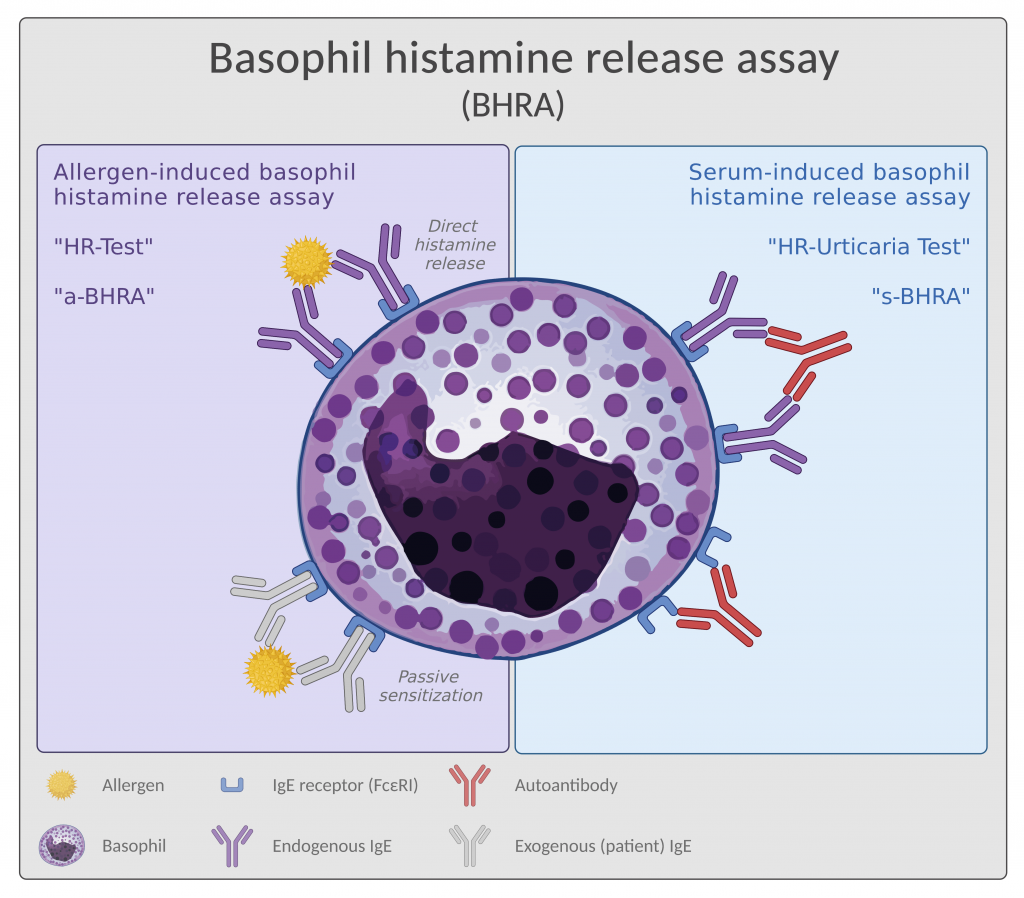
The assay setup differs slightly dependent on which indication it is beeing used for. Therefore we call it allergen-induced BHRA (a-BHRA) or serum-induced BHRA (s-BHRA) as seen in Figure 1 below.
The allergen-induced BHRA
(a-BHRA)
The allergen-induced BHRA (a-BHRA) is also called the Histamine Release Test (HR-Test). In this version of BHRA, sensitized basophils are challenged with the suspected allergen to detect functional IgE, which can be measured as histamine release from the basophils (Figure 1, left side).
a-BHRA can be performed with the patient’s own basophils in a full blood sample (measuring a direct histamine release) or with donor basophils sensitized with IgE from a patient serum sample (measuring histamine release after passive sensitization). The former is more sensitive, making it the preferred option, but both versions of a-BHRA detect sensitization towards an allergen.
The serum-induced BHRA
(s-BHRA)
The serum-induced BHRA (s-BHRA) is also called the HR-Urticaria Test. In this version of the assay, donor basophils are challenged with patient serum to detect the presence of activating auto-antibodies (Figure 1, right side) characteristic of a specific sub-type of chronic spontaneous urticaria (CSU), namely type IIb.
